A Year Ago, Ghana Approved Oxford’s R21 Malaria Vaccine
Last Updated
12th April, 2025
Date Published
11th April, 2025
Share This Post With Someone

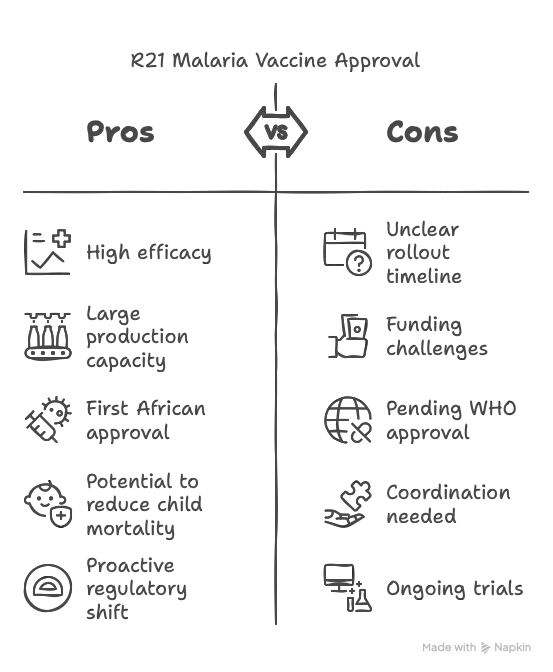
- Historic Approval: Ghana is the first nation to approve Oxford University’s R21 malaria vaccine, a breakthrough in the fight against malaria, which kills hundreds of thousands of children yearly.
- Approval Timing: The approval on April 13, 2023, preceded the publication of final-stage trial data, an unusual move as it awaits further evaluation by bodies like the World Health Organization (WHO).
- Regulatory Authority: Ghana’s Food and Drugs Authority (FDA), led by CEO Delese Darko, granted the approval, emphasizing its role as a regulator independent of WHO’s support.
- Target Group: The vaccine is approved for children aged 5 to 36 months, the demographic most vulnerable to malaria-related deaths.
- Vaccine Details: Named R21, the vaccine is produced by the Serum Institute of India, with a capacity to manufacture up to 200 million doses annually.
- Global Significance: This marks the first time a major vaccine was approved in an African country before wealthier nations, reflecting a shift in regulatory proactivity in Africa post-COVID.
- Trial Data: Mid-stage trials with over 400 children showed 70-80% efficacy at 12 months after the fourth dose. Phase III trials with 4,800 children in Burkina Faso, Kenya, Mali, and Tanzania are ongoing, with results expected soon.
- Comparison with Mosquirix: The R21 vaccine is the second malaria vaccine approved, following GSK’s Mosquirix, which has been rolled out in Ghana, Kenya, and Malawi since 2019, reaching 1.2 million children and reducing child mortality by 10% in vaccinated areas.
- Production Advantage: Unlike Mosquirix, limited to 15 million doses annually by GSK, R21’s production scale offers a solution to meet the WHO’s estimated need of 100 million doses yearly for 25 million children.
- Challenges: The rollout timeline in Ghana remains unclear, pending coordination with the Ghana Health Service, Malaria Programme, and immunization body. Funding and WHO approval are critical for broader distribution.
- African Regulatory Shift: African regulators, spurred by COVID-era delays, are adopting a proactive stance to avoid being last in accessing critical health interventions.
- Malaria Burden: Malaria kills over 600,000 people annually, mostly African children, highlighting the urgency of effective vaccines.
- Development History: The complex malaria parasite has challenged vaccine development for decades, making R21’s high efficacy a notable achievement.
- WHO’s Role: While WHO is assessing R21’s safety and effectiveness, it does not approve vaccines but supports regulatory processes, leaving final decisions to national authorities like Ghana’s FDA.
Key Terms:
- R21 Vaccine: Oxford’s malaria vaccine with 70-80% efficacy, approved for young children.
- Malaria: Mosquito-borne disease causing over 600,000 deaths yearly, primarily in Africa.
- Ghana FDA: Ghana’s regulatory body that approved R21, showcasing African regulatory leadership.
- Serum Institute: Indian manufacturer producing up to 200 million R21 doses annually.
- Mosquirix: GSK’s malaria vaccine, first approved, with limited production capacity.
- WHO: Global health body assessing R21, not an approving authority but a key influencer.
- Phase III Trials: Ongoing large-scale tests of R21 involving 4,800 African children.
- Efficacy: R21’s 70-80% effectiveness in preventing malaria after four doses.