UPSC
The Hindu Briefs
Indigenous TB Test: Enhancing Accuracy and Speed
Last Updated
13th April, 2025
Date Published
13th April, 2025
Share This Post With Someone

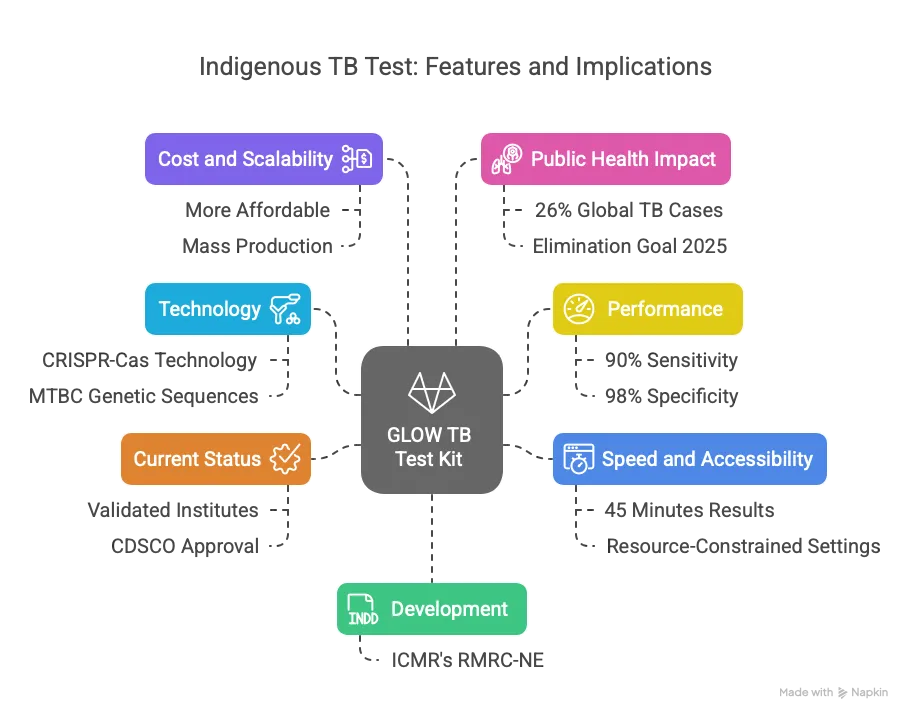
- Development by ICMR:
- The ICMR’s Regional Medical Research Centre for North East (RMRC-NE) in Dibrugarh developed a CRISPR-Cas-based TB detection kit.
- Named ‘GLOW’, it is India’s first indigenous TB diagnostic tool, reducing reliance on imported kits.
- Technology:
- The kit uses CRISPR-Cas technology to detect the Mycobacterium tuberculosis complex (MTBC) in sputum samples.
- It targets specific MTBC genetic sequences for accurate identification.
- Performance:
- Offers 90% sensitivity (ability to detect true positives) and 98% specificity (ability to avoid false positives).
- Outperforms conventional methods like smear microscopy (less sensitive) and matches nucleic acid amplification tests (NAATs) in accuracy.
- Speed and Accessibility:
- Delivers results in approximately 45 minutes, compared to hours for other molecular tests.
- Designed for use in resource-constrained settings, requiring minimal training and equipment.
- Current Status:
- Validated across four ICMR institutes in Dibrugarh, Bhubaneswar, Chennai, and New Delhi.
- Awaiting approval from the Central Drugs Standard Control Organisation (CDSCO) for commercial production.
- Cost and Scalability:
- Expected to be more affordable than imported kits, enhancing access in rural and remote areas.
- ICMR plans to transfer technology to industry partners for mass production post-approval.
- Public Health Impact:
- India accounts for 26% of global TB cases, with 2.7 million new cases annually.
- Early and accurate diagnosis can reduce transmission and improve treatment outcomes.
- Supports India’s goal to eliminate TB by 2025, ahead of the global 2030 target.
- Additional Features:
- Capable of detecting multi-drug resistant TB (MDR-TB), critical for addressing resistant strains.
- Potential to integrate with existing TB diagnostic platforms for wider adoption.
Key Terms:
- CRISPR-Cas: Gene-editing technology used for precise detection of TB bacteria.
- Mycobacterium tuberculosis: Bacterium causing TB, targeted by the GLOW kit.
- Sensitivity: Ability of a test to correctly identify TB-positive cases.
- Specificity: Ability of a test to correctly identify TB-negative cases.
- MDR-TB: Multi-drug resistant tuberculosis, resistant to standard treatments.
- ICMR: Indian Council of Medical Research, leading medical research in India.
- CDSCO: Central Drugs Standard Control Organisation, India’s drug regulatory body.
Link To The Original Article – https://www.thehindu.com/sci-tech/indigenous-tb-test-can-enhance-accuracy-speed-up-testing/article69439931.ece